From Wikipedia, the free encyclopedia
Health education is the profession of educating people about health. Areas within this profession encompass environmental health, physical health, social health, emotional health, intellectual health, and spiritual health. It can be defined as the principle by which individuals and groups of people learn to behave in a manner conducive to the promotion, maintenance, or restoration of health. However, as there are multiple definitions of health, there are also multiple definitions of health education. The Joint Committee on Health Education and Promotion Terminology of 2001 defined Health Education as “any combination of planned learning experiences based on sound theories that provide individuals, groups, and communities the opportunity to acquire information and the skills needed to make quality health decisions.” The World Health Organization defined Health Education as “compris[ing] [of] consciously constructed opportunities for learning involving some form of communication designed to improve health literacy, including improving knowledge, and developing life skills which are conducive to individual and community health.”
|
Contents
- 1 The Role of the Health Educator
- 2 Motivation
- 3 Credentialing
- 4 Teaching
- 5 National Health Education Standards
- 6 Health Education Code of Ethics
- 7 Health Education Code of Ethics Full Text
- 8 National Organizations for Public Health/Health Education
- 9 Health Education Career Opportunities
- 10 Influential Individuals in Health Education: Past and Present
- 11 See also
- 12 References
- 13 External links
|
The Role of the Health Educator
From the late nineteenth to the mid-twentieth century, the aim of public health was controlling the harm from infectious diseases, which were largely under control by the 1950s. By the mid 1970s it was clear that reducing illness, death, and rising health care costs could best be achieved through a focus on health promotion and disease prevention. At the heart of the new approach was the role of a health educator A health educator is “a professionally prepared individual who serves in a variety of roles and is specifically trained to use appropriate educational strategies and methods to facilitate the development of policies, procedures, interventions, and systems conducive to the health of individuals ,groups, and communities” (Joint Committee on Terminology, 2001, p. 100). In January 1979 the Role Delineation Project was put into place, in order to define the basic roles and responsibilities for the health educator. The result was a Framework for the Development of Competency-Based Curricula for Entry Level Health Educators (NCHEC, 1985). A second result was a revised version of A Competency-Based Framework for the Professional Development of Certified Health Education Specialists (NCHEC,1996). These documents outlined the seven areas of responsibilities which are shown below 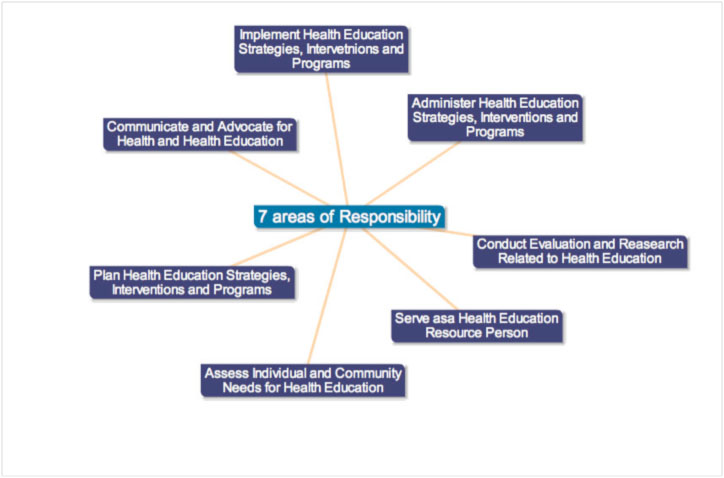 .EDZ
.EDZ
Responsibility I: Assessing Individual and Community Needs for Health Education
* Provides the foundation for program planning * Determines what health problems might exist in any given group * Includes determination of community resources available to address the problem * Community Empowerment encourages the population to take ownership of their health problems * Includes careful data collection and analysis
Responsibility II: Plan Health Education Strategies, Interventions, and Programs
* Actions are based on the needs assessment done for the community (see Responsibility I) * Involves the development of goals and objectives which are specific and measurable * Interventions are developed that will meet the goals and objectives * According to Rule of Sufficiency, strategies are implemented which are sufficiently robust, effective enough, and have a reasonable chance of meeting stated objectives
Responsibility III: Implement Health Education Strategies, Interventions, and Programs
* Implementation is based on a thorough understanding of the priority population * Utilize a wide range of educational methods and techniques
Responsibility IV: Conduct Evaluation and Research Related to Health Education
* Depending on the setting, utilize tests, surveys, observations, tracking epidemiological data, or other methods of data collection * Health Educators make use of research to improve their practice
Responsibility V: Administer Health Education Strategies, Interventions, and Programs
* Administration is generally a function of the more experienced practitioner * Involves facilitating cooperation among personnel, both within and between programs
Responsibility VI: Serve as a Health Education Resource Person
* Involves skills to access needed resources, and establish effective consultive relationships
Responsibility VII: Communicate and Advocate for Health and Health Education
* Translates scientific language into understandable information * Address diverse audience in diverse settings * Formulates and support rules, policies and legislation * Advocate for the profession of health education
Motivation
Education for health begins with people. It hopes to motivate them with whatever interests they may have in improving their living conditions. Its aim is to develop in them a sense of responsibility for health conditions for themselves as individuals, as members of families, and as communities. In communicable disease control, health education commonly includes an appraisal of what is known by a population about a disease, an assessment of habits and attitudes of the people as they relate to spread and frequency of the disease, and the presentation of specific means to remedy observed deficiencies.
Health education is also an effective tool that helps improve health in developing nations. It not only teaches prevention and basic health knowledge but also conditions ideas that re-shape everyday habits of people with unhealthy lifestyles in developing countries. This type of conditioning not only affects the immediate recipients of such education but also future generations will benefit from an improved and properly cultivated ideas about health that will eventually be ingrained with widely spread health education. Moreover, besides physical health prevention, health education can also provide more aid and help people deal healthier with situations of extreme stress, anxiety, depression or other emotional disturbances to lessen the impact of these sorts of mental and emotional constituents, which can consequently lead to detrimental physical effects. ,
Credentialing
Credentialing is the process by which the qualifications of licensed professionals, organizational members or an organization are determined by assessing the individuals or group background and legitimacy through a standardized process. Accreditation, licensure, or certifications are all forms of credentialing.
In 1978, Helen Cleary, the president of the Society for Public Health Education (SOPHE) started the process of certification of health educators. Prior to this, there was no certification for individual health educators, with exception to the licensing for school health educators. The only accreditation available in this field was for school health and public health professional preparation programs.
Her initial response was to incorporate experts in the field and to promote funding for the process. The director if the Division of Associated Health Professions in the Bureau of Health Manpower of the Department of Health, Education, and Welfare, Thomas Hatch, became interested in the project. To ensure that the commonalities between health educators across the spectrum of professions would be sufficient enough to create a set of standards, Dr. Cleary spent a great amount of time to create the first conference called the Bethesda Conference. In attendance were interested professionals who covered the possibility of creating credentialing within the profession.
With the success of the conference and the consensus that the standardization of the profession was vital, those who organized the conference created the National Task Force in the Preparation and Practice of Health Educators. Funding for this endeavor became available in January 1979, and role delineation became a realistic vision for the future. They presented the framework for the system in 1981 and published entry-level criteria in 1983. Seven areas of responsibility, 29 areas of competency and 79 sub-competencies were required of health education professionals for approximately 20 years for entry-level educators.
In 1986 a second conference was held in Bethesda, Maryland to further the credentialing process. In June 1988, the National Task Force in the Preparation and Practice of Health Educators became the National Commission for Health Education Credentialing, Inc. (NCHEC). Their mission was to improve development of the field by promoting, preparing and certifying health education specialists. The NCHEC has three division boards that included preparation, professional development and certification of health educator professionals. The third board, which is called the Division Board of Certification of Health Education Specialist (DBCHES), has the responsibility of developing and administering the CHES exam. An initial certification process allowed 1,558 individuals to be chartered into the program through a recommendation and application process. The first exam was given in 1990.
In order for a candidate to sit for a exam they must have either a bachelor’s, master’s, or doctoral degree from and accredited institution, and an official transcript that shows a major in health education, Community Health Education, Public Health Education, or School Health Education, etc. The transcript will be accepted if it reflects 25 semester hours or 37 quarter hours in health education preparation and covers the 7 responsibilities covered in the framework.
In 1998 a project called the Competencies Update Project (CUP) began. The purpose of the CUP project was to up-date entry-level requirements and to develop advanced-level competences. Through research the CUP project created the requirements for three levels, which included entry-level, Advanced I and Advanced II educators.
Recently the Master Certified Health Education Specialist (MCHES) is in the process of being created. It is an exam that will measure the knowledge of the advanced levels and sub levels of the Seven Areas of Responsibilities. The first MCHES exam is expected to be given in October of 2011.
In order to be eligible to take the MCHES exam you must have at least a Master's degree in health education or related discipline along with a least 25 credit hours related to health education. In addition, five years of documented information of practice in health education and two recommendations of past/present supervisors must be provided. A vitae/resume must also be submitted.
The Competency Update Project (CUP), 1998-2004 revealed that there were higher levels of health education practitioners, which is the reasoning for the advancements for the MCHES. Many health educators felt that the current CHES credential was an entry-level exam.
There will be exceptions made for those who have the Certification of Health Education Specialist, that have been active for several consecutive years. They will be required to participate in the MCHES Experience Documentation Opportunity that will omit them from taking the exam.
Teaching
In the United States some forty states require the teaching of health education. A comprehensive health education curriculum consists of planned learning experiences which will help students achieve desirable attitudes and practices related to critical health issues. Some of these are: emotional health and a positive self image; appreciation, respect for, and care of the human body and its vital organs; physical fitness; health issues of alcohol, tobacco, drug use and abuse; health misconceptions and myths; effects of exercise on the body systems and on general well being; nutrition and weight control; sexual relationships and sexuality, the scientific, social, and economic aspects of community and ecological health; communicable and degenerative diseases including sexually transmitted diseases; disaster preparedness; safety and driver education; factors in the environment and how those factors affect an individual's or population's Environmental health (ex: air quality, water quality, food sanitation); life skills; choosing professional medical and health services; and choices of health careers.
National Health Education Standards
The National Health Education Standards (NHES) are written expectations for what students should know and be able to do by grades 2, 5, 8, and 12 to promote personal, family, and community health. The standards provide a framework for curriculum development and selection, instruction, and student assessment in health education. The performance indicators articulate specifically what students should know or be able to do in support of each standard by the conclusion of each of the following grade spans: Pre-K–Grade 2; Grade 3–Grade 5; Grade 6–Grade 8; and Grade 9–Grade 12. The performance indicators serve as a blueprint for organizing student assessment.
| Standard 1 |
Standard 2 |
Standard 3 |
Standard 4 |
Standard 5 |
Standard 6 |
Standard 7 |
Standard 8 |
| Students will comprehend concepts related to health promotion and disease prevention to enhance health. |
Students will analyze the influence of family, peers, culture, media, technology, and other factors on health behaviors. |
Students will demonstrate the ability to access valid information, products, and services to enhance health. |
Students will demonstrate the ability to use interpersonal communication skills to enhance health and avoid or reduce health risks. |
Students will demonstrate the ability to use decision-making skills to enhance health. |
Students will demonstrate the ability to use goal-setting skills to enhance health. |
Students will demonstrate the ability to practice health-enhancing behaviors and avoid or reduce health risks. |
Students will demonstrate the ability to advocate for personal, family, and community health. |
| Performance Indicators for Pre-K-Grade 2 |
Performance Indicators for Pre-K-Grade 2 |
Performance Indicators for Pre-K-Grade 2 |
Performance Indicators for Pre-K-Grade 2 |
Performance Indicators for Pre-K-Grade 2 |
Performance Indicators for Pre-K-Grade 2 |
Performance Indicators for Pre-K-Grade 2 |
Performance Indicators for Pre-K-Grade 2 |
| 1.2.1 Identify that healthy behaviors impact personal health.
1.2.2 Recognize that there are multiple dimensions of health.
1.2.3 Describe ways to prevent communicable diseases.
1.2.4 List ways to prevent comes.
1.2.5 Describe why it is important to seek health care.
|
2.2.1 Identify how the family influences personal health practices and behaviors.
2.2.2 Identify what the school can do to support personal health practices and behaviors.
2.2.3 Describe how the media can influence health behaviors.
|
3.2.1 Identify trusted adults and professionals who can help promote health.
3.2.2 Identify ways to locate school and community health helpers.
|
4.2.1 Demonstrate healthy ways to express needs, wants, and feelings.
4.2.2 Demonstrate listening skills to enhance health.
4.2.3 Demonstrate ways to respond in an unwanted, threatening, or dangerous situation.
4.2.4 Demonstrate ways to tell a trusted adult if threatened or harmed.
|
5.2.1 Identify situations when a health-related decision is needed.
5.2.2 Differentiate between situations when a health-related decision can be made individually or when assistance is needed.
|
6.2.1 Identify a short-term personal health goal and take action toward achieving the goal.
6.2.2 Identify who can help when assistance is needed to achieve a personal health goal.
|
7.2.1 Demonstrate healthy practices and behaviors to maintain or improve personal health.
7.2.2 Demonstrate behaviors that avoid or reduce health risks.
|
8.2.1 Make requests to promote personal health.
8.2.2 Encourage peers to make positive health choices.
|
| Performance Indicators for Grades 3-5 |
Performance Indicators for Grades 3-5 |
Performance Indicators for Grades 3-5 |
Performance Indicators for Grades 3-5 |
Performance Indicators for Grades 3-5 |
Performance Indicators for Grades 3-5 |
Performance Indicators for Grades 3-5 |
Performance Indicators for Grades 3-5 |
| 1.5.1 Describe the relationship between healthy behaviors and personal health.
1.5.2 Identify examples of emotional, intellectual, physical, and social health.
1.5.3 Describe ways in which safe and healthy school and community environments can promote personal health.
1.5.4 Describe ways to prevent common childhood injuries and health problems.
1.5.5 Describe when it is important to seek health care.
|
2.5.1 Describe how family influences personal health practices and behaviors.
2.5.2 Identify the influence of culture on health practices and behaviors.
2.5.3 Identify how peers can influence healthy and unhealthy behaviors
2.5.4 Describe how the school and community can support personal health practices and behaviors.
2.5.5 Explain how media influences thoughts, feelings, and health behaviors.
2.5.6 Describe ways that technology can influence personal health.
|
3.5.1 Identify characteristics of valid health information, products, and services.
3.5.2 Locate resources from home, school, and community that provide valid health information.
|
4.5.1 Demonstrate effective verbal and nonverbal communication skills to enhance health.
4.5.2 Demonstrate refusal skills that avoid or reduce health risks.
4.5.3 Demonstrate nonviolent strategies to manage or resolve conflict.
4.5.4 Demonstrate how to ask for assistance to enhance personal health.
|
5.5.1 Identify health-related situations that might require a thoughtful decision.
5.5.2 Analyze when assistance is needed in making a health-related decision.
5.5.3 List healthy options to health-related issues or problems.
5.5.4 Predict the potential outcomes of each option when making a health-related decision.
5.5.5 Choose a healthy option when making a decision.
5.5.6 Describe the outcomes of a health-related decision.
|
6.5.1 Set a personal health goal and track progress toward its achievement.
6.5.2 Identify resources to assist in achieving a personal health goal.
|
7.5.1 Identify responsible personal health behaviors.
7.5.2 Demonstrate a variety of healthy practices and behaviors to maintain or improve personal health.
7.5.3 Demonstrate a variety of behaviors to avoid or reduce health risks.
|
8.5.1 Express opinions and give accurate information about health issues.
8.5.2 Encourage others to make positive health choices.
|
| Performance Indicators for Grades 6-8 |
Performance Indicators for Grades 6-8 |
Performance Indicators for Grades 6-8 |
Performance Indicators for Grades 6-8 |
Performance Indicators for Grades 6-8 |
Performance Indicators for Grades 6-8 |
Performance Indicators for Grades 6-8 |
Performance Indicators for Grades 6-8 |
| 1.8.1 Analyze the relationship between healthy behaviors and personal health.
1.8.2 Describe the interrelationships of emotional, intellectual, physical, and social health in adolescence.
1.8.3 Analyze how the environment affects personal health.
1.8.4 Describe how family history can affect personal health.
1.8.5 Describe ways to reduce or prevent injuries and other adolescent health problems.
1.8.6 Explain how appropriate health care can promote personal health.
1.8.7 Describe the benefits of and barriers to practicing healthy behaviors.
1.8.8 Examine the likelihood of injury or illness if engaging in unhealthy behaviors.
1.8.9 Examine the potential seriousness of injury or illness if engaging in unhealthy behaviors.
|
2.8.1 Examine how the family influences the health of adolescents.
2.8.2 Describe the influence of culture on health beliefs, practices, and behaviors.
2.8.3 Describe how peers influence healthy and unhealthy behaviors.
2.8.4 Analyze how the school and community can affect personal health practices and behaviors.
2.8.5 Analyze how messages from media influence health behaviors.
2.8.6 Analyze the influence of technology on personal and family health.
2.8.7 Explain how the perceptions of norms influence healthy and unhealthy behaviors.
2.8.8 Explain the influence of personal values and beliefs on individual health practices and behaviors.
2.8.9 Describe how some health risk behaviors can influence the likelihood of engaging in unhealthy behaviors.
2.8.10 Explain how school and public health policies can influence health promotion and disease prevention.
|
3.8.1 Analyze the validity of health information, products, and services.
3.8.2 Access valid health information from home, school, and community.
3.8.3 Determine the accessibility of products that enhance health.
3.8.4 Describe situations that may require professional health services.
3.8.5 Locate valid and reliable health products and services.
|
4.8.1 Apply effective verbal and nonverbal communication skills to enhance health.
4.8.2 Demonstrate refusal and negotiation skills that avoid or reduce health risks.
4.8.3 Demonstrate effective conflict management or resolution strategies.
4.8.4 Demonstrate how to ask for assistance to enhance the health of self and others.
|
5.8.1 Identify circumstances that can help or hinder healthy decision making.
5.8.2 Determine when health-related situations require the application of a thoughtful decision-making process.
5.8.3 Distinguish when individual or collaborative decision making is appropriate.
5.8.4 Distinguish between healthy and unhealthy alternatives to health-related issues or problems.
5.8.5 Predict the potential short-term impact of each alternative on self and others.
5.8.6 Choose healthy alternatives over unhealthy alternatives when making a decision.
5.8.7 Analyze the outcomes of a health-related decision.
|
6.8.1 Assess personal health practices.
6.8.2 Develop a goal to adopt, maintain, or improve a personal health practice.
6.8.3 Apply strategies and skills needed to attain a personal health goal.
6.8.4 Describe how personal health goals can vary with changing abilities, priorities, and responsibilities.
|
7.8.1 Explain the importance of assuming responsibility for personal health behaviors.
7.8.2 Demonstrate healthy practices and behaviors that will maintain or improve the health of self and others. 7.8.3 Demonstrate behaviors to avoid or reduce health risks to self and others.
|
8.8.1 State a health-enhancing position on a topic and support it with accurate information.
8.8.2 Demonstrate how to influence and support others to make positive health choices.
8.8.3 Work cooperatively to advocate for healthy individuals, families, and schools.
8.8.4 Identify ways in which health messages and communication techniques can be altered for different audiences.
|
| Performance Indicators for Grades 9-12 |
Performance Indicators for Grades 9-12 |
Performance Indicators for Grades 9-12 |
Performance Indicators for Grades 9-12 |
Performance Indicators for Grades 9-12 |
Performance Indicators for Grades 9-12 |
Performance Indicators for Grades 9-12 |
Performance Indicators for Grades 9-12 |
| 1.12.1 Predict how healthy behaviors can affect health status.
1.12.2 Describe the interrelationships of emotional, intellectual, physical, and social health.
1.12.3 Analyze how environment and personal health are interrelated.
1.12.4 Analyze how genetics and family history can impact personal health.
1.12.5 Propose ways to reduce or prevent injuries and health problems.
1.12.6 Analyze the relationship between access to health care and health status.
1.12.7 Compare and contrast the benefits of and barriers to practicing a variety of healthy behaviors.
1.12.8 Analyze personal susceptibility to injury, illness, or death if engaging in unhealthy behaviors.
1.12.9 Analyze the potential severity of injury or illness if engaging in unhealthy behaviors.
|
2.12.1 Analyze how the family influences the health of individuals.
2.12.2 Analyze how the culture supports and challenges health beliefs, practices, and behaviors.
2.12.3 Analyze how peers influence healthy and unhealthy behaviors.
2.12.4 Evaluate how the school and community can affect personal health practice and behaviors.
2.12.5 Evaluate the effect of media on personal and family health.
2.12.6 Evaluate the impact of technology on personal, family, and community health.
2.12.7 Analyze how the perceptions of norms influence healthy and unhealthy behaviors.
2.12.8 Analyze the influence of personal values and beliefs on individual health practices and behaviors.
2.12.9 Analyze how some health risk behaviors can influence the likelihood of engaging in unhealthy behaviors.
2.12.10 Analyze how public health policies and government regulations can influence health promotion and disease prevention.
|
3.12.1 Evaluate the validity of health information, products, and services.
3.12.2 Use resources from home, school, and community that provide valid health information.
3.12.3 Determine the accessibility of products and services that enhance health.
3.12.4 Determine when professional health services may be required.
3.12.5 Access valid and reliable health products and services.
|
4.2.1 Demonstrate healthy ways to express needs, wants, and feelings.
4.12.1 Use skills for communicating effectively with family, peers, and others to enhance health.
4.12.2 Demonstrate refusal, negotiation, and collaboration skills to enhance health and avoid or reduce health risks.
4.12.3 Demonstrate strategies to prevent, manage, or resolve interpersonal conflicts without harming self or others.
4.12.4 Demonstrate how to ask for and offer assistance to enhance the health of self and others.
|
5.12.1 Examine barriers that can hinder healthy decision making.
5.12.2 Determine the value of applying a thoughtful decision-making process in health-related situations.
5.12.3 Justify when individual or collaborative decision making is appropriate.
5.12.4 Generate alternatives to health-related issues or problems.
5.12.5 Predict the potential short-term and long-term impact of each alternative on self and others.
5.12.6 Defend the healthy choice when making decisions.
5.12.7 Evaluate the effectiveness of health-related decisions.
|
6.12.1 Assess personal health practices and overall health status.
6.12.2 Develop a plan to attain a personal health goal that addresses strengths, needs, and risks.
6.12.3 Implement strategies and monitor progress in achieving a personal health goal.
6.12.4 Formulate an effective long-term personal health plan.
|
7.12.1 Analyze the role of individual responsibility for enhancing health.
7.12.2 Demonstrate a variety of healthy practices and behaviors that will maintain or improve the health of self and others.
7.12.3 Demonstrate a variety of behaviors to avoid or reduce health risks to self and others.
|
8.12.1 Utilize accurate peer and societal norms to formulate a health-enhancing message.
8.12.2 Demonstrate how to influence and support others to make positive health choices.
8.12.3 Work cooperatively as an advocate for improving personal, family, and community health.
8.12.4 Adapt health messages and communication techniques to a specific target audience.
|
Health Education Code of Ethics
The Health Education Code of Ethics has been a work in progress since approximately 1976, begun by the Society of Public Health Education (SOPHE). Various Public Health and Health Education organizations such as the American Association of Health Education (AAHE), the Coalition of National Health Education Organizations (CNHEO), SOPHE, and others collaborated year after year to devise a unified standard of ethics that health educators would be held accountable to professionally. In 1995, the National Commission for Health Education Credentialing, Inc. (NCHEC) proposed a profession-wide standard at the conference: Health Education Profession in the Twenty-First Century: Setting the Stage. Post-conference, an ethics task force was developed with the purpose of solidifying and unifying proposed ethical standards. The document was eventually unanimously approved and ratified by all involved organizations in November 1999 and has since then been used as the standard for practicing health educators.
“The Code of Ethics that has evolved from this long and arduous process is not seen as a completed project. Rather, it is envisioned as a living document that will continue to evolve as the practice of Health Education changes to meet the challenges of the new millennium.”
Health Education Code of Ethics Full Text
PREAMBLE The Health Education profession is dedicated to excellence in the practice of promoting individual, family, organizational, and community health. The Code of Ethics provides a framework of shared values within which Health Education is practiced. The responsibility of each Health Educator is to aspire to the highest possible standards of conduct and to encourage the ethical behavior of all those with whom they work.
Article I: Responsibility to the Public A Health Educator’s ultimate responsibility is to educate people for the purpose of promoting, maintaining, and improving individual, family, and community health. When a conflict of issues arises among individuals, groups, organizations, agencies, or institutions, health educators must consider all issues and give priority to those that promote wellness and quality of living through principles of self-determination and freedom of choice for the individual.
Article II: Responsibility to the Profession Health Educators are responsible for their professional behavior, for the reputation of their profession, and for promoting ethical conduct among their colleagues.
Article III: Responsibility to Employers Health Educators recognize the boundaries of their professional competence and are accountable for their professional activities and actions.
Article IV: Responsibility in the Delivery of Health Education Health Educators promote integrity in the delivery of health education. They respect the rights, dignity, confidentiality, and worth of all people by adapting strategies and methods to the needs of diverse populations and communities.
Article V: Responsibility in Research and Evaluation Health Educators contribute to the health of the population and to the profession through research and evaluation activities. When planning and conducting research or evaluation, health educators do so in accordance with federal and state laws and regulations, organizational and institutional policies, and professional standards.
Article VI: Responsibility in Professional Preparation Those involved in the preparation and training of Health Educators have an obligation to accord learners the same respect and treatment given other groups by providing quality education that benefits the profession and the public.
All versions of the document are available on the Coalition of National Health Education's site: http://www.cnheo.org/. The National Health Education Code of Ethics is the property of the Coalition of National Health Education.
National Organizations for Public Health/Health Education
American Public Health Association (APHA) APHA is the main voice for public health advocacy that is the oldest organization of public health sine 1872. The American Public Health Association aims to “protect all Americans and their communities from preventable, serious health threats and strives to assure community-based health promotion and disease preventions.” Any individual can become a member and benefit in online access and monthly printed issues of The Nation’s Health and the American Journal of Public Health
Society for Public Health Education (SOPHE) The mission of SOPHE is to provide global leadership to the profession of health education and health promotion and to promote the health of society through advances in health education theory and research, excellence in professional preparation and practice, and advocacy for public policies conducive to health, and the achievement of health equity for all. Membership is open to all who have an interest in health education and or work in health education in schools, medical care settings, worksites, community based organizations, state/local government, and international agencies. Founded in 1950, SOPHE publishes 2 indexed, peer-reviewed journals, Health Education & Behavior and Health Promotion Practice.
American School Health Association (ASHA) The American School Health Association was founded in 1972 by a group of physicians that already belonged to the American Public Health Association. This group specializes in school-aged health specifically. Over the years it has snowballed and now includes any person that can be a part of a child’s life, from dentists, to counselors and school nurses. The American School Health Association mission “is to protect and promote the health of children and youth by supporting coordinated school health programs as a foundation for school success.”
American Association of Health Education/American Alliance for Health, Physical Education, Recreation, and Dance (AAHE/AAHPERD) The AAHE/AAHPERD is said to be the largest organization of professionals that supports physical education; which includes leisure, fitness, dance, and health promotion. That is only a few; this incorporates all that is physical movement. This organization is an alliance with five national associations and six districts and is there to provide a comprehensive and coordinated array of resources to help support practitioners to improve their skills and always be learning new things. This organization was first stated in November 1885. William Gilbert Anderson had been out of medical school for two years and was working with many other people that were in the gymnastic field. He wanted them to get together to discuss their field and this organization was created. Today AAHPERD serves 25,000 members and has its headquarters in Reston, Virginia.
Eta Sigma Gamma (ESG) The Eta Sigma Gamma is a national health education organization founded in 1967 by three professor from Ball tate University. The mission of the ESG to promote public health education by improving the standards, ideals, capability, and ethics of public health education professionals. The three key points of the organization are to teach, research, and provide service to the members of the public health professionals. Some of the goals that the Eta Stigma Gamma targets are support planning and evaluation of future and existing health education programs, support and promote scientific research, support advocacy of health education issues, and promote professional ethics.
American College Health Association (ACHA) The American College Health Association originally began as a student health association in 1920, but then in 1948 the association changed the name to what its known today. The principal interest of the ACHA is to promote advocacy and leadership to colleges and universities around the country. Other part of the mission's association is to encourage education, communication, and services to students and campus community in general. The association also promotes advocacy and research. The American College Health Association has three types of membership: institutions of higher education, individual members who are interested in the public health profession, and susbtain members which are profitable and non-profitable organization. The ACHA is connected to 11 organizations located in six regions around the country. Currently, the American College Health Association serves 900 educative institutions and about 2400 individual members in the United States.
Directors of Health Promotion and Education (DHPE) Founded in 1946 as one of the professional groups of the Health Education Profession. The main goal of the HEPE is to improve the health education standards in any public health agency. As well, build networking opportunities among all public health professionals as a media to communicate ideas for implementing health programs, and to keep accurate information about the latest health news. The DHPE also focus to increase public awareness of health education and promotion by creating and expanding methods of existing health programs that will improve the quality of health. The Directors of Health Promotion and Education is linked to the Association of State and Territorial Health Officials (ASTHO) to “work on health promotion and disease prevention”.
Health Education Career Opportunities
The terms Public Health Educator, Community Health Educator or Health Educator are all used interchangeable to describe an individual who plans implements and evaluates health education and promotion programs. These individuals play a crucial role in many organizations in various settings to improve our nations health. Just as a Community health educator works work toward population health, a school Health educator generally teaches in our Schools. A community health educator is typically focused on their immediate community striving to serve the public.
Health Care Settings: these include hospitals (for-profit and public), medical care clinics, home health agencies, HMOs and PPOs. Here, a health educator teaches employees how to be healthy. Patient education positions are far and few between because insurance companies do not cover the costs. [1]
Public Health Agencies: are official, tax funded, government agencies. They provide police protection, educational systems, as well as clean air and water. Public health departments provide health services and are organized by a city, county, state, or federal government. [2]
School Health Education: involves all strategies, activities, and services offered by, in, or in association with schools that are designed to promote students' physical, emotional, and social development. School health involves teaching students about health and health related behaviors. Curriculum and programs are based on the school's expectations and health. [3]
Non Profit Voluntary Health Agencies: are created by concerned citizens to deal with health needs not met by governmental agencies. Missions include public education, professional education, patient education, research, direct services and support to or for people directly affected by a specific health or medical problem. Usually funded by such means as private donations, grants, and fund-raisers.[4]
Higher Education: typically two types of positions health educators hold including academic, or faculty or health educator in a student health service or wellness center. As a faculty member, the health educator typically has three major responsibilities: teaching, community and professional service, and scholarly research. As a health educator in a university health service or wellness center, the major responsibility is to plan, implement, and evaluate health promotion and education programs for program participants. [5]
Work site Health Promotion: is a combination of educational, organizational and environmental activities designed to improve the health and safety of employees and their families. These work site wellness programs offer an additional setting for health educators and allow them to reach segments of the population that are not easily reached through traditional community health programs. Some work site health promotion Some work site health promotion activities include; smoking cessation, stress management, bulletin boards, newsletters, and much more. [6]
Independent Consulting and Government Contracting: international, national, regional, sate, and local organizations contract with independent consultants for many reasons. They may be hired to assess individual and community needs for health education; plan, implement, administer and evaluate health education strategies; conduct research; serve as health education resource person; and or communicate about and advocate for health and health education. Government contractors are often behind national health education programs, government reports, public information web sites and telephone lines, media campaigns, conferences, and health education materials. [7]
Influential Individuals in Health Education: Past and Present
Dorothy Bird Nyswander
Dr. Nyswander was born on Sept. 29, 1894. She earned her Bachelor's and Master's degree at the University of Nevada and received her Doctorate in educational psychology at Berkeley. She is a founder of the School of Public Health at the University of California at Berkeley. Dr. Nysawnder pursued her interest in public health at the Works Progress Administration during the depression. She served with the Federal Works Agency contributing to the establishment of nursery schools and child care centers to accommodate young mothers working in defense plants. She set up these centers in 15 northeastern states. This did not happen quickly so she advocated all over the nation to train people to act as foster parents for the children of working women. Dr. Nyswander became the director of the City health Center in Astoria Queens in 1939. She spent her time as director promoting the idea of New York City keeping an eye on the health of children. They would do this by keeping records that would follow them to whatever school they might move to. She wrote “Solving School Health Problems” which is an analysis of the health issues in New York children. This is still used in public health education courses today.
Mayhew Derryberry
Dr. Derryberry was born December 25, 1902 and earned his Bachelor's degree in chemistry and mathematics at the University of Tennessee. He began his career in 1926 with the American Child Health Association as the director of one of the first large-scale studies of the health status of the nation’s schoolchildren. A year after his work with the American Child Health Association he earned his Master's degree in education and psychology at Columbia University. He then went on to earn his doctorate and moved to the New York City Health Department as the secretary to the sanitary superintendent. He finally moved to Washington DC and joined the US Public Health Service as a senior public health analyst. He became chief of the Public Health Service and began assembling a team of behavioral scientists. They studied the nexus of behavior, social factors, and disease. Two scientists and Derryberry conducted the study of the role of health beliefs in explaining utilization of public health screening services. This work contributed to the development of the Health Belief Model. This provided an important theoretical foundation for modern health education. His legacy was very important because he engaged behavioral and social scientists in the problems of public health and gave importance to the role of that health education plays on human health.
Elena Sliepcevich
Elena Sliepcevich was a leading figure in the development of health education both as an academic discipline and a profession. She graduated from the University of Ireland in 1939 and received her Master's degree from the University of Michigan in 1949. She received her doctorate in physical education from Springfield College in 1955. After completing her schooling, Elena Sliepcevich worked at Ohio State University in 1961 as a professor of health education. There she helped direct the School Health Education Study from 1961-1969, and most health education curricula used in schools today are based on the ten conceptual areas identified by the School Health Education Study. These ten areas of focus include community health, consumer health, environmental health, family life, mental and emotional health, injury prevention and safety, nutrition, personal health, prevention and control of disease, and drug use and abuse.
Helen Agnes Cleary
Helen Cleary was born March 28, 1914 at Petersburg, South Australia. She trained as a nurse at the Broken Hill and District Hospital in New South Wales. She became a general nurse in 1941, and an obstetric nurse in 1942. She joined the Royal Australian Air Force Nursing Service as a sister on November 15, 1943. Along with other RAAF nurses, she would partake in evacuations throughout New Guinea and Borneo, which earned the nurses the nickname “the flying angels”, and were also known as the “glamor girls” of the air force. In April 1945, she was ranked No. 2 Medical Air Evacuation Transport Unit, and began bringing thousands of Australian and British servicemen from prisoner-of-war camps after Japan had surrendered. She and other nurses cared for many patients who suffered from malnutrition and dysentery. During the Korean War, Cleary was charge sister on the RAAF, where she organized medical evacuations of Australians from Korea, fought for better treatment and conditions of the critically wounded, and nursed recently exchanged Prisoners of War. On August 18, 1967, Ms. Cleary was made honorary nursing sister to Queen Elizabeth II. She had been appointed an associate of the Royal Red Cross in 1960, and became a leading member in 1968 for her contributions to the training of medical staff, and for maintaining “the high ideals of the nursing profession”. She retired on March 28, 1969, and later died on August 26, 1987.
Delbert Oberteuffer
A long time health educator, Delbert Oberteuffer definitely made his mark on the physical education and health education world. He was born in Portland, Oregon in 1902 where he remained through college, attending the University of Oregon receiving his Bachelors Degree. His next step took him to the prestigious Columbia University where he obtained his Masters of Arts and Doctor of Philosophy Degree. He furthered his education by becoming a professor at Ohio State University where he taught from 1932 until 1966. During his time there, he was head of the Men's Physical Education Department for 25 years. After years of hard work, he was rewarded with numerous jobs including the President of the American School Health Association and The College of Physical Education Association. Unfortunately, he passed away in 1981 at the age of 79. He is Survived by his wife, Katherine, and his son, Theodore K. Oberteuffer.
Howard Hoyman
Howard Hoyman is mainly recognized for his work in sex education and introductions of ecology concepts. He is credited for developing the original sex education program for students in grades 1 through 12. The model Hoyman created heavily influenced the thinking of many health educators. Hoyman received his Bachelors Degree from Ohio State University in 1931. He then went on to earn his Masters degree in 1932 and Doctorate in 1945 from the University of Colombia. Throughout his career he wrote over 200 articles and was honored many times by multiple organizations such as Phi Beta Kappa and the American Public Health Association. Dr. Hoyman retired in 1970 as A Professor Emeritus.
Lloyd Kolbe
Lloyd Kolbe received his B.S. form Towson University and then received his Ph.D. and M.Ed. from the University of Toledo during the 1970’s. Dr. Kolbe played a huge role in the development of many health programs applied to the daily life of different age groups. He received the award for Excellence in Prevention and Control of Chronic Disease, which is the highest recognition in his department of work, for his work forming the Division of Adolescent and School Health. Dr. Kolbe was the Director of this program for 15 years. He has also taken time to write and publish numerous books such as Food marketing to Children and Youth and School as well as Terrorism Related to Advancing and Improving the Nation’s Health.
Robert Morgan Pigg
University of Florida professor, Robert Morgan Pigg, started his health career in 1969 when he received his Bachelors Degree in Health, Physical Education, and Recreation from Middle Tennessee State University. A year later he received his M.Ed; also from Middle Tennessee University before moving on to Indian University where he obtained his H.S.D. in 1974 and his M.P.H. in 1980. He held many jobs at numerous Universities including Western Kentucky University, University of Georgia, Indiana University, and the University of Florida where he currently resides today. Pigg's main focus of interest is the promotion of health towards children and adolescents. After spending 20 years as Editor for the Journal of Health, he was given the job as Department Chair in 2007 for The University of Florida.
Linda Rae Murray
Linda Rae Murray holds her MD, and MPH. Currently she is the Chief Officer for the Ambulatory & Community Health Network. She was elected president November 2009. Dr. Murray has served in a number of Medical settings her most recent being Medical Director of the federally funded health center, Winfield Moody, serving the Cabrini Green public housing project in Chicago. She has also been an active member of the board of national organizations. Along with this she served as Chief Medical Officer in primary care for the twenty three primary care and community health centers. Today Murray serves as the Chief Medical Officer for the Cook County Health & Hospital system. Dr. Murray has also been a voice for social justice and health care as a basic human right for over forty years.
Mark J. Kittleson
Mark J. Kittleson is a professor at Southern Illinois University for Public Health Education. His interests include Educational Technology and Behaviorism; he attended the University of Akron and received his PhD in Health Education. Dr. Kittleson has experience as owner and founder of the HEDIR a place where people can hold discussions related to health and health education. His honors and awards consist of Scholar of the Year, American Association of Health Education 2008 and he is a member of the American Association of Health Education.
Elaine Auld
Elaine Auld has been a leading figure for over more than 30 years in the health education field. She attended the University of Michigan, MPH, and Health Behavior/Health Education, from 1976 – 1978 Elaine is the chief executive officer for the Society for Public Health Education (SOPHE) and has had many contributions in health promotion and health communications. She has been a certified health specialist since 1989 and in 1996 was an adviser to the first Health Education Graduate Standards. Elaine was involved with the Competency Update Project (CUP), which provided standards for the health education profession. Elaine’s interest and work are related to health education credentialing and standards, workforce development, public policy, and health equity. For the last decade Elaine has been a site visitor for the Council on Education for Public Health, and also strengthened the accreditation and preparation of future health specialists, which is key to an overall healthy well-being. Elaine has received two awards U of MI SPH Alumni of the Year Award in 2010 and SOPHE Distinguished Fellow in 2008.
Susan Wooley
Susan Wooley received her bachelor’s degree from Case Western Reserve University, a master’s degree in health education from the University of North Carolina at Greensboro, and a Ph.D. in health education from Temple University. Susan is the executive director of the American School Health Association and has been a member to ASHA for 31 years. She co-edited Health Is Academic: A Guide to Coordinated School Health Programs and co-authored Give It a Shot, a Toolkit for Nurses and Other Immunization Champions Working with Secondary Schools. Susan has had many previous jobs such as CDC’s Division of Adolescent and School Health, Delaware State College, American Association for Health Education and Delaware Department of Public Instruction and is also a certified health specialist. Wooley spent four years on a curriculum development project for elementary schools, Science for Life and Living: Integrating Science, Technology and Health. Now Susan oversees the day-to-day operations of a national professional association and provides consultation and technical assistance to others working toward health education.
See also
- Dairy Council of California
- Dorothy Nyswander
- Environmental Health
- Health Literacy
- Health Promotion
- Health Teacher
- Healthy People 2010
- Life skills
- Online health communities
- Personal, Social and Health Education
- Physical Education
- Public Health
- School Health Education Study
- AAHPERD
References
- ^ McKenzie, J., Neiger, B., Thackeray, R. (2009). Health Education and Health Promotion. Planning, Implementing, & Evaluating Health Promotion Programs. (pp. 3-4). 5th edition. San Francisco, CA: Pearson Education, Inc.
- ^ Donatelle, R. (2009). Promoting Healthy Behavior Change. Health: The basics. (pp. 4). 8th edition. San Francisco, CA: Pearson Education, Inc.
- ^ Joint Committee on Terminology. (2001). Report of the 2000 Joint Committee on Health Education and Promotion Terminology. American Journal of Health Education, 32(2), 89-103.
- ^ World Health Organization. (1998). List of Basic Terms. Health Promotion Glossary. (pp. 4). Retrieved May 1, 2009 from http://www.who.int/hpr/NPH/docs/hp_glossary_en.pdf.
- ^ Cottrell,Girvan,and McKenzie, 2009.
- ^ Washington State Department of Health
- ^ Bundy, D., Guya, H.L. (1996). Schools for health, education and the school-age child. Parasitology Today, 12(8), 1-16.
- ^ Kann, L., Brener, N.D., Allensworth, D.D. (2001). Health education: Results from the School Health Policies and Programs Study 2000. Journal of School Health, 71(7), 266-278.
- ^ Cottrell, R. R., Girvan, J. T., & McKenzie, J. F. (2009). Principles and Foundations of Health Promotion and Education. New York: Benjamin Cummings.
- ^ Patterson, S. M., & Vitello, E. M. (2006). Key Influences Shaping Health Education: Progress Toward Accreditaion. The Health Education Monograph Series, 23(1), 14- 19.
- ^ [1]
- ^ Centers for Disease Control & Prevention. (2007). National Health Education Standards. Retrieved May 1, 2009 from http://www.cdc.gov/HealthyYouth/SHER/standards/index.htm
- ^ Coalition of National Health Education Organizations. Introduction. Health Education Code of Ethics. November 8, 1999, Chicago, IL. Retrieved May 1, 2009 from http://www.cnheo.org/code1.pdf
- ^ Coalition of National Health Education Organizations. Introduction. Health Education Code of Ethics. November 8, 1999, Chicago, IL. Retrieved May 1, 2009 from http://www.cnheo.org/code3.pdf
- ^ [2]
- ^ [3]
- ^ [4]
- ^ [5]
- ^ [6]
- ^ [7]
- ^ [8]
- ^ [9]
- ^ [10]
- ^ [11]
- ^ [12]
- ^ [13]
- ^ [14]
- ^ [15]
- ^ [16]
- ^ [17]
- ^ [18]
- ^ [19]
- ^ [20]
- ^ [21]
- Centers for Disease Control & Prevention. (2007). National Health Education Standards. Retrieved May 1, 2009 from http://www.cdc.gov/HealthyYouth/SHER/standards/index.htm
- Coalition of National Health Education Organizations. Health Education Code of Ethics. November 8, 1999, Chicago, IL. Retrieved May 1, 2009 from http://www.cnheo.org
- Donatelle, R. (2009). Health: The basics. 8th edition. San Francisco, CA: Pearson Education, Inc.
- Joint Committee on Terminology. (2001). Report of the 2000 Joint Committee on Health Education and Promotion Terminology. American Journal of Health Education.
- McKenzie, J., Neiger, B., Thackeray, R. (2009). Planning, Implementing, & Evaluating Health Promotion Programs. 5th edition. San Francisco, CA: Pearson Education, Inc.
- Simons-Morton, B. G. , Greene, W. H., & Gottlieb, N. H.. (2005). Introduction to Health Education and Health Promotion. 2nd edition. Waveland Press.
- World Health Organization. (1998). Health Promotion Glossary. Retrieved May 1, 2009 from http://www.who.int/hpr/NPH/docs/hp_glossary_en.pdf.
External links
- Health Education through Animation Video
- Association of Academic Health Centers (USA)
- Association of Canadian Academic Healthcare Organizations (Canada)
- British Columbia Academic Health Council (Canada)
- Centers for Disease Control and Prevention (USA)
- American Association for Health Education (United States)
- National Commission for Health Education Credentialing, Inc. (USA)
- Association of Schools of Public Health (USA)
- US Department of Health & Human Services (USA)
- American Public Health Association (USA)
- National Institutes of Health (USA)
- World Health Organization (USA)
- Sexuality Information and Education Council of the United States (USA)
- Environmental Protection Agency (USA)
- American School Health Association (USA)
- Society of State Directors of Health, Physical Education, and Recreation (USA)
- National Health Education Standards (USA)

 .EDZ
.EDZ
